

Intravacc is at the forefront of vaccine innovation and development for infectious diseases, antimicrobial resistance (AMR), and oncology leveraging our proprietary Outer Membrane Vesicle (OMV) platform.
Outer Membrane Vesicles (OMVs) are naturally occurring, spherical particles, typically ranging in size from 20 to 200 nanometers. They are released by gram-negative bacteria and contain bacterial antigens.


Read our latest publication about our Outer Membrane Vesicle (OMV) platform: an insightful interview with our VP of R&D, Dinja Oosterhoff, PhD
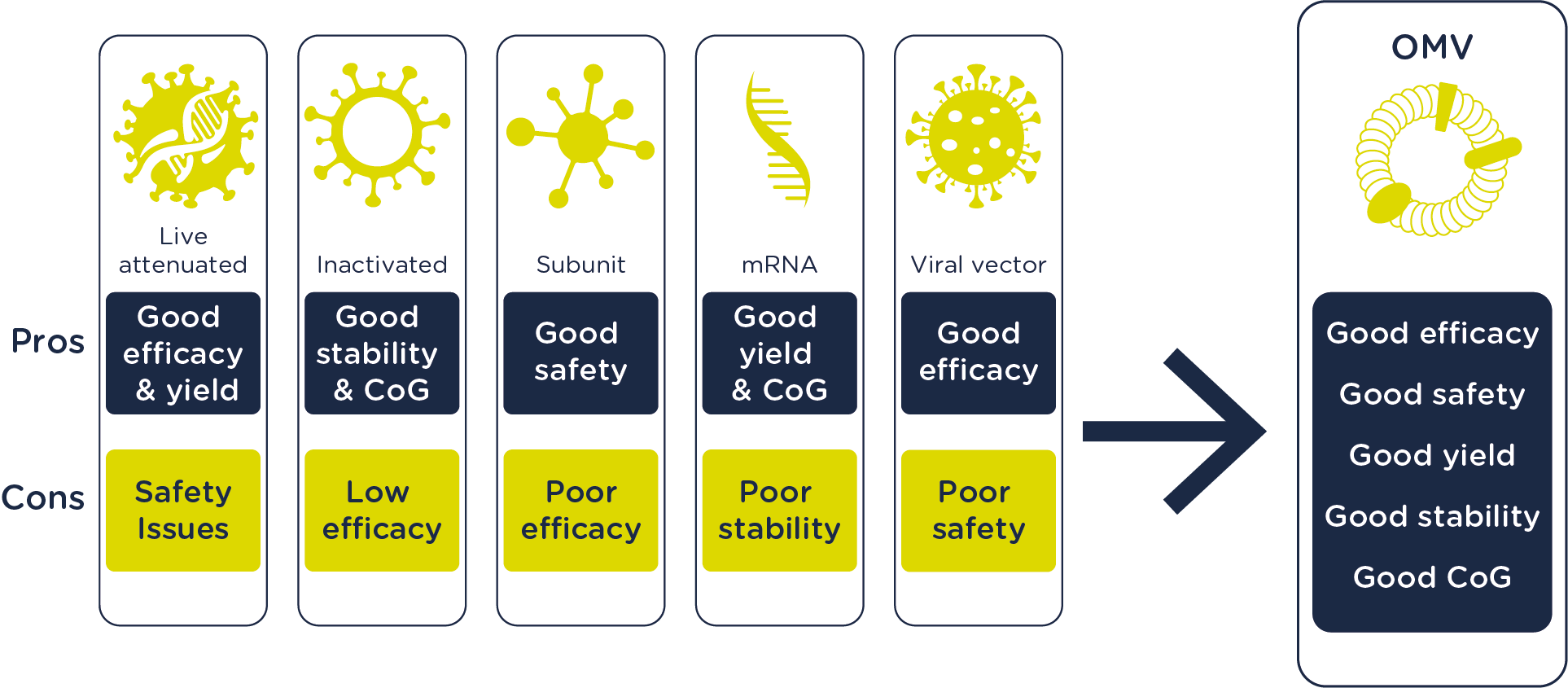

We back our OMV technology with expertise demonstrated through 84 scientific publications in peer-reviewed journals and 8 patent families since 2013. We've developed genetic tools to increase OMV yield, minimize toxicity, and tailor antigenic composition. Our scalable platform allows efficient antigen composition modifications through genetic manipulation or association with carrier OMVs. Several of our vaccines under development build on the OMV platform. Currently, we are actively working on the development of a vaccine for gonorrhea and COVID-19.
See which of our vaccine candidates build on OMV-Vacc.
Intravacc offers three types of OMV-based vaccines, each designed to target specific diseases and antigens:
Intravacc is your trusted partner for OMV-based vaccine development. Our focus is on providing accurate, evidence-based solutions for scientists and researchers dedicated to addressing infectious diseases.
Learn more about our OMV platform.
Discuss your project with one of our experts.



“Worldwide resurgence of whooping cough calls for improved, next-generation pertussis vaccines that induce broad and long-lasting immunity. A pertussis vaccine based on outer membrane vesicles is a promising candidate. We have successfully in-licensed the pertussis OMV technology from Intravacc recently. Our efforts are focused on advancing this innovative vaccine technology through the clinical pipeline, with the aim of combatting the resurgence of whooping cough using Pertussis Vaccine based on OMV technology”.
Picture:The team of Dr. Yao Lei (in the middle) and the team of Intravacc during the technology transfer at Intravacc in Bilthoven, The Netherlands, in June 2023.

From idea to GMP manufacturing, implement any of our production platforms with the support of our tailored CDMO services.
You can send us an email:
info@intravacc.nl
Reach out to Business Development:
BD@intravacc.nl
Or pick up the phone:
+31 30 792 03 00
You can also just fill out the contact form on the right.
We look forward to hearing from you!
