

Here you can learn from partner and client voices why working with our team has been a positive and successful experience. In the following case studies, some of our partnering and clients projects are briefly described. Moreover you can find links to press releases and other information about the project.


Infection by enterovirus D68 represents a global public health concern. EV D68 is a respiratory virus that can cause childhood paralysis, AFM and asthma-related illnesses. First described in 1962, EV D68 circulated sporadically until August 2014 when outbreaks were reported in the USA and Canada mainly under children with AFM and severe acute respiratory disease. EV D68 is considered a priority pathogen by Centers for Disease Control and Prevention (CDC).
In 2020, Intravacc was awarded a 9.4 million USD contract from the US National Institutes of Health (NIH) to develop a prophylactic vaccine against EV D68. Intravacc is developing an inactivated EV D68 vaccine, based on its proprietary Vero cell technology, from early product selection through to phase I clinical testing.
Gonorrhea is caused by a Neisseria gonorrhoeae infection. Left untreated, gonorrhea leads to reproductive health complications. Infections during pregnancy are associated with chorioamnionitis, preterm birth, spontaneous abortions, and other problems. Furthermore, the bacterium exhibits antimicrobial resistance, hindering treatment.
Intravacc was awarded a contract from the US National Institute of Allergy and Infectious Diseases (NIAID) to develop a prophylactic intranasal vaccine against N. gonorrhoeae. The vaccine candidate, called Avacc 11, combines our OMV-Vacc technology with sustained-release microspheres containing recombinant human IL-12. Intravacc has developed a complete production process under GMP, and the vaccine will be tested in a phase I clinical trial.



Since outbreak of the COVID-19 pandemic, the SARS-CoV-2 genome has rapidly mutated, resulting in numerous SARS-CoV-2 variants. Some of these variants are highly transmissible, cause more severe disease, or evade protection from therapeutics and/or vaccines. These have been designated Variants of Concern.
Avacc® 10, our intranasal OMV-based SARS-CoV-2 booster vaccine candidate, induces local mucosal immunity and a systemic response at the primary entry route of SARS-CoV-2 is targeted, potentially reducing viral replication at an early stage and blocking transmission. Avacc 10 has entered phase I first in-human clinical trial to demonstrate safety and efficacy. Under an additional funding award from the Coalition for Epidemic Preparedness Innovations (CEPI), we are also working to develop vaccines that provide broad protection against SARS-CoV-2, including its variants, and other Betacoronaviruses.
Amyotrophic lateral sclerosis (ALS) is an uncommon, advancing, and fatal neurodegenerative condition resulting in the depletion of motor neurons in the brain and spinal cord. Presently, patients face a life expectancy of 3-5 years post-diagnosis, with limited treatment options available. Approximately 5-10% of individuals of European descent diagnosed with ALS carry a mutation known as C9orf72, leading to the accumulation of neurotoxic repeat proteins. Intravacc and the German Center of Neurodegenerative Diseases (DZNE) collaborate under the EU-funded GA-VAX project to develop a preventative and therapeutic vaccine specifically tailored for this ALS subtype. The vaccine candidate initiates an immune response against the neurotoxic protein, demonstrating promising results in averting and addressing neurodegeneration in a mouse model. Currently progressing towards a First-in-Human phase I/II clinical study, this groundbreaking candidate marks a significant stride towards combating ALS.



Whooping cough, or pertussis, is a highly contagious respiratory disease caused by the gram-negative bacterium Bordetella pertussis. While all ages can be infected, infants are the main risk group. Worldwide there are an estimated 24 million cases.
Current pertussis vaccines have limitations. Some are relatively reactogenic, while others show limited efficacy. Our Avacc 3 induces a strong systemic and mucosal immune response when administered intranasally. Beijing Zhifei Lvzhu Biopharmaceutical Co. received an exclusive license for the Chinese territory for our Avacc 3. We are collaborating to advance Avacc 3 through upscaling, toxicology, and clinical trials to commercialize the vaccine for the Chinese market.
Meningitis B is an uncommon but serious disease caused by a bacterial infection in the lining of the brain and spinal cord. It can also cause meningococcal septicemia. Liaoning Chengda Biotechnology CO., Ltd, (CDBIO) has an ongoing collaboration with Intravacc to advance a vaccine against Meningococcus type B built on our OMV-Vacc platform. Both parties collaborate to tailor the vaccine for the Chinese market and CDBIO will receive a license to commercialize it.



Prevention Coxsackie virus B (CVB) has been identified in the pancreas of around 60% of individuals with type 1 diabetes. Persistent CVB infection is significantly linked to the onset of both type 1 diabetes and celiac disease.
The pentavalent inactivated CVB vaccine, RV-101, emerges as a potential solution for acute CVB infection, potentially preventing up to 50% of type 1 diabetes cases and approximately 20% of celiac disease cases. Developed and produced for ProventionBio, a US company, the clinical trial material for PRV-101 has yielded positive results in ProventionBio's first-in-human PROVENT study.
Cimcure, a Dutch company, has pinpointed protein targets in tumor vasculature to eradicate endothelial cells, disrupting blood supply to cancer cells and impeding tumor growth. Their vaccine, featuring the vimentin target protein conjugated to a proprietary engineered bacterial sequence, induces robust antibody production upon administration. These antibodies, in turn, stimulate an immune response against tumor blood vessels. Demonstrating proof of concept in colon cancer, melanoma, and glioblastoma, Cimcure collaborates with Intravacc for CDMO services, utilizing the E.co-Vacc technology to advance the vaccine's development.



Shigellosis, a disease involving diarrhea and gastrointestinal symptoms, results from the ingestion of Shigella flexneri 2a and causes approximately 250 million cases annually in low- and middle-income countries.
In a collaborative effort, Intravacc partnered with the Institut Pasteur Paris in the EU-funded Stopenterics project (European Community's Seventh Framework Programme, FP7/2007-2013). This collaboration aimed to develop a Good Manufacturing Practice (GMP)-grade scalable production process for manufacturing material for a conjugate vaccine against shigellosis. This material was intended for a phase I clinical trial, testing the vaccine candidate. The phase I clinical trial proved successful, leading to two ongoing phase II studies — a de-escalating age study in Kenya and a Controlled Human Infection Model study in the USA.
One in 200 infections with poliovirus leads to irreversible paralysis. Among those paralyzed, 5–10% die when their breathing muscles become immobilized. In January 2021, Intravacc announced the WHO pre-qualification for the Sabin-IPV (sIPV) vaccine Eupolio™. The vaccine was developed by Intravacc for technology transfer to manufacturers in low- and middle-income countries in the context of the global polio eradication initiative.
The South Korean company LG Chem signed a contract with UNICEF to supply 80 million USD worth of polio vaccine Eupolio. Since 2012, the company supplies the vaccine to 70 countries in the Middle East, Africa and Southeast Asia. In 2021, the Chinese National Medical Products Administration granted Sinovac Biotech LTD market authorization for its Sabin-IPV (sIPV) inactivated polio vaccine, which was developed by Intravacc and out-licensed to Sinovac. Our technology was also transferred to the Beijing company Minhai Biotechnology, and their sIPV vaccine is currently in phase II trials.
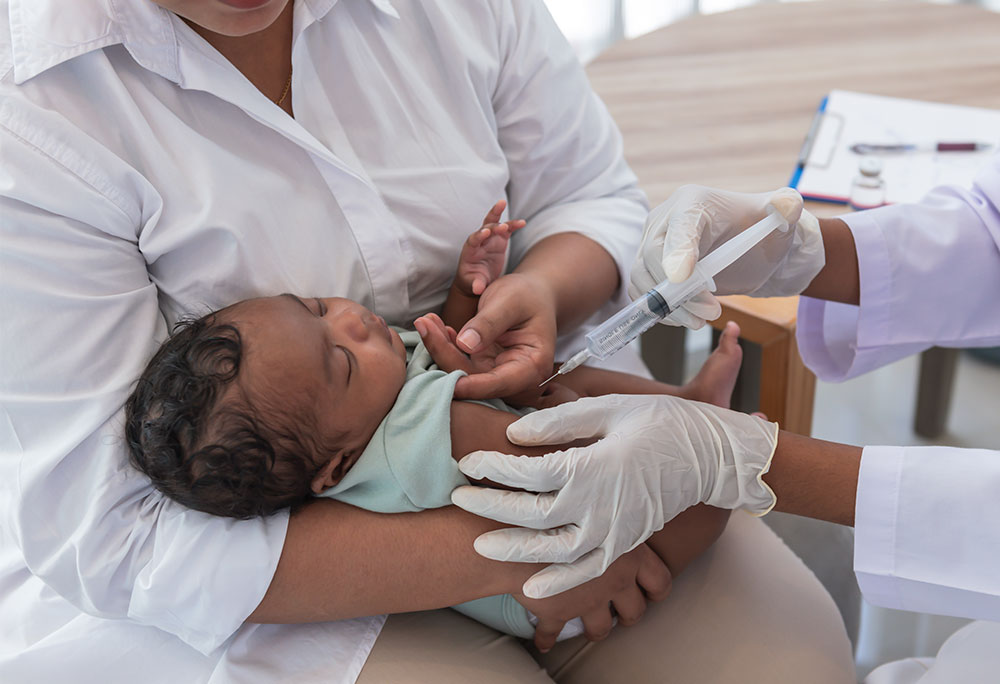

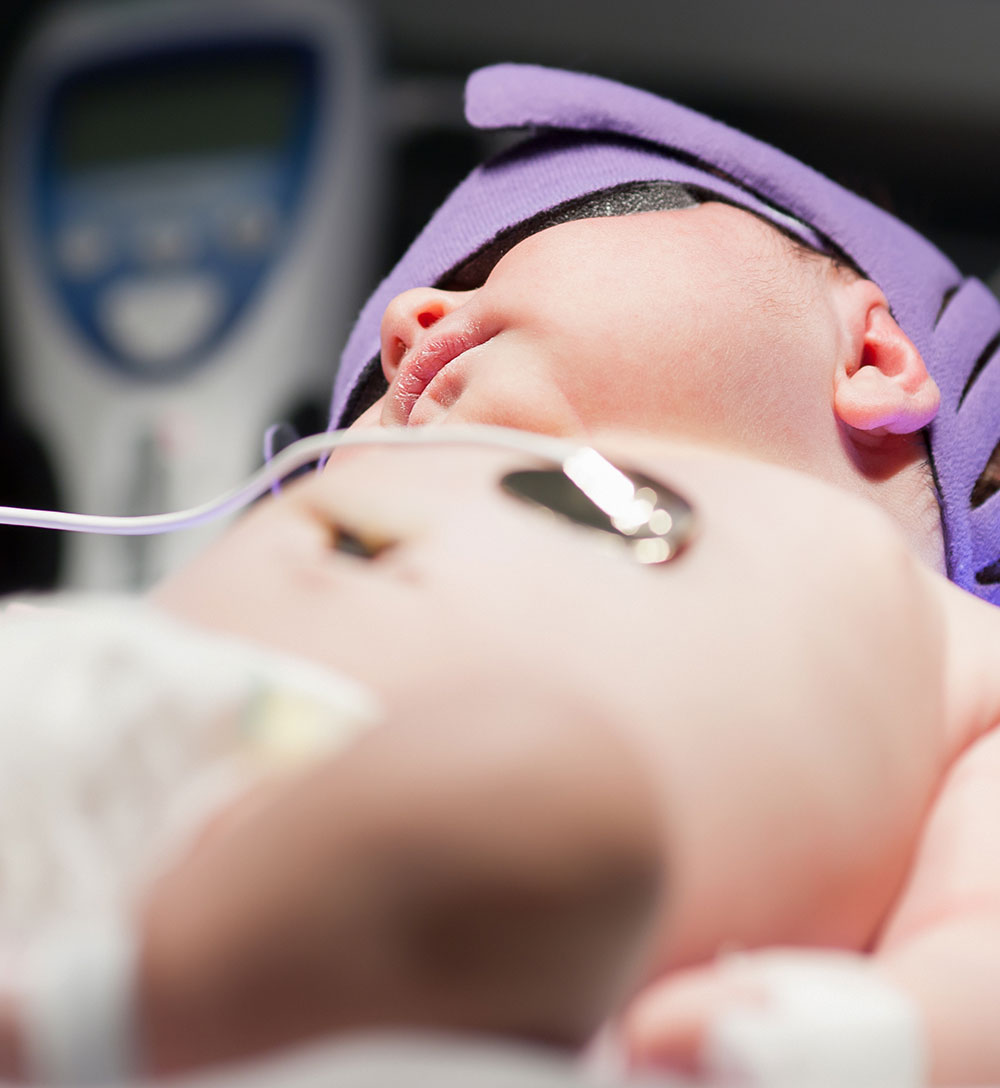

Haemophilus influenzae Type B, or Hib, is a bacterium that can cause life-threatening infections, especially in infants and children. In 2000, the WHO estimated that Hib was responsible for 8.13 million cases of serious illness and over 370,000 deaths worldwide. Intavacc and Changchun BCHT Biotechnology Co (BCHT) entered into a collaboration to develop a Hib conjugate vaccine, consisting of a Hib polysaccharide (PRP) coupled to tetanus toxoid, within the People’s Republic of China. In the partnership, BCHT obtained Intravacc’s unique Hib conjugate technology to further develop, produce, and sell the vaccine. The Intravacc also works with the Serum Institute of India, the world’s largest vaccine manufacturer, to make its WHO-prequalified Hib vaccine available to millions of children.
You can send us an email:
info@intravacc.nl
Reach out to Business Development:
BD@intravacc.nl
Or pick up the phone:
+31 30 792 03 00
You can also just fill out the contact form on the right.
We look forward to hearing from you!
