

Vaccines have revolutionized public health, helping to control, reduce, and even eliminate many serious diseases thereby advancing public health goals. Creating a new vaccine, however, is a complicated process that can take 10 to 15 years.1 Intravacc has a strong track record in vaccine development and technology transfer, with highlights such as the Sabin inactivated polio (sIPV), Haemophilus influenza type B (Hib), and the Bordatella pertussis vaccines.
1 International Federation of Pharmaceutical Manufacturers & Associations. 2019. "The complex journey of a vaccine: the steps behind developing a new vaccine."
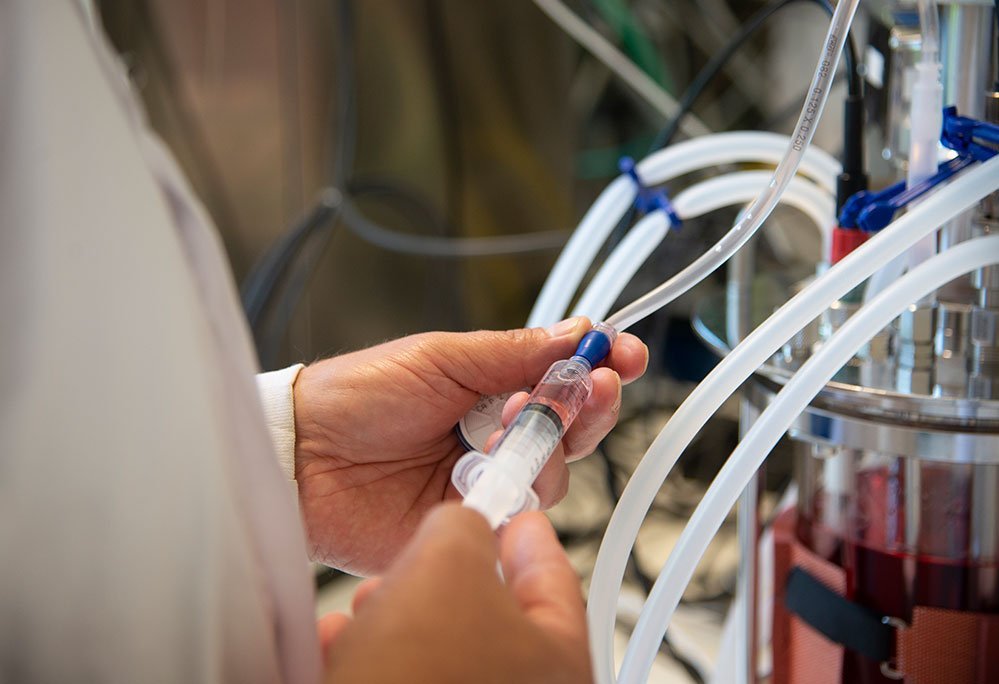
Working with partners worldwide, Intravacc carries out independent vaccine development from lead concept to phase II clinical studies. We design and develop new vaccines, improve the manufacturing processes of existing vaccines, and produce drug substances and products at cGMP-level to enable clinical studies.
Intravacc develops vaccines via established blueprints and scalable platform production processes. These processes are rationally designed using mathematical models to meet regulatory requirements and to be scalable from lab to production-scale bioreactors. Both processes and their products are tested in our state-of-the-art laboratory facilities using the latest ICH guidelines and insights.


Our quality and regulatory affairs experts are involved at an early stage in our projects. They ensure regulatory compliance and help provide feasible solutions to challenging project needs in partnership with other company divisions.
You can send us an email:
info@intravacc.nl
Reach out to Business Development:
BD@intravacc.nl
Or pick up the phone:
+31 30 792 03 00
You can also just fill out the contact form on the right.
We look forward to hearing from you!
