Process Development
USP & DSP
Vaccine strain design and development
Modern vaccine development takes advantage of advances in technology and scientific understanding of the immune system and host-pathogen interactions. At Intravacc, the creation of a vaccine begins with viral or bacterial strain design.
Modern vaccine development takes advantage of advances in technology and scientific understanding of the immune system and host-pathogen interactions. At Intravacc, the creation of a vaccine begins with viral or bacterial strain design.
Our flexible technologies for development of bacterial vaccines includes inactivated bacteria, bacterial toxins, conjugate vaccines, and our proprietary Outer Membrane Vesicles (OMV) platform.
For therapeutic vaccine candidates we take advantages of our existing vaccine platforms Cell based, OMV and Conjugate vaccine to develop therapeutic vaccines.
We select vaccine antigens via bioinformatics or forced evolution, in silico design, and subsequent construction. In this process, we optimize safety, immunogenicity (such as level of expression and epitope design), intrinsic antigen stability, intrinsic adjuvant safety (LPS) and vaccine yield.
To ensure GMP readiness, we genetically modify our bacterial strains without selection markers in the final production strain. Our viral strains are carefully vetted for purity and quality criteria. Finally, we emphasize production processes that are free of animal components.
Excellence in robust and optimized process development
During production, numerous materials, including biologically sourced materials, are combined in a series of process steps to produce a final vaccine. In the upstream process (USP), the biological source organism is amplified to make a crude harvest. In the downstream process (DSP), the crude intermediate product – typically recombinant antigens or entire pathogens – is purified to yield a bulk vaccine product. The final formulation completes the production process by, for example, combining antigens and adjuvant into a defined stable formulation for long-term storage.
Intravacc’s manufacturing specialists have developed scalable upstream and downstream biomanufacturing processes with cells, viruses, and bacteria for over 20 years. With expertise in a multitude of production processes, our team is ready to optimize your vaccine development.
Upstream processing (USP)

Intravacc’s expertise in upstream processing covers fully characterized, optimized, and scalable viral propagation and cultivation of bacterial and eukaryotic cells in flasks and bioreactors of up to 50 L and 200 L respectively. Our team is also experienced in advanced molecular techniques and adherent cell culture on microcarriers at various scales, thereby covering a broad range of techniques to realize any vaccine concept.
Client-dedicated process facilities
Our process development facilities are designed to accommodate dedicated long-term development and characterization programs. With focused and customized support, we create a cost-efficient and robust development process. From cell and seed lot banking to medium design, batch, fed-batch, or continuous cultures, our goal is to increase the yield and viability of your vaccine culture.
Key features of our services and facilities include:
- Top-of-the-line cell culture equipment
- Adherent and suspension cell lines
- Bacterial cultivation
- High-throughput process development
- Design of Experiments (DOE)
- Scales from 5 mL up to 200 L
- Glass, stainless steel, and single-use bioreactors
Downstream processing (DSP)
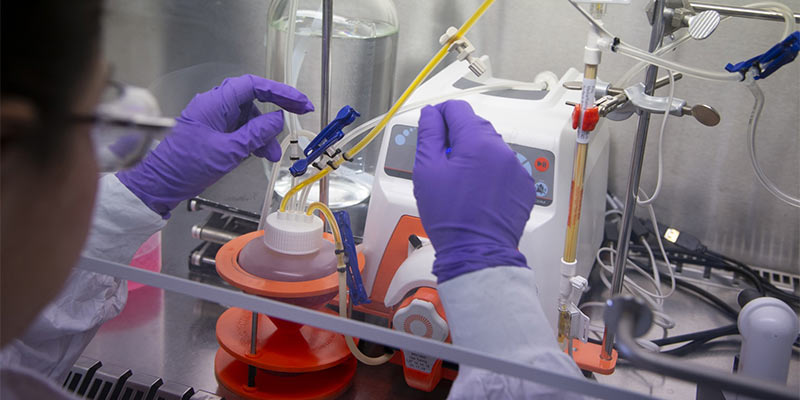
Efficiency is the goal of Intravacc’s downstream process (DSP) development. Using a broad range of technologies, our team designs robust procedures to obtain consistently pure and high-quality products from biological substances and maintain efficient target antigen recovery.
The right technology for each project
From filtration (NFF/TFF), gradient centrifugation, ultracentrifugation, and selective precipitation to chromatographic separations and viral inactivation, we use the best and most cost-effective methods for development and optimization at lab, pilot, or production scale. In addition, our experience working with our flexible platforms allows us to maximize target recovery in protein, membrane, and viral products.
Key features of our technological portfolio include:
- Homogenization
- Filtration NFF/TFF, (incl. MF, UF, DF)
- Gradient centrifugation and ultracentrifugation
- Chromatographic separations
- Fully scalable
- Single-use technology
Optimal formulations for effective vaccines
Vaccines generally combine components to achieve high bioavailability and bioactivity while eliciting a desired type and degree of immune stimulation.
In the final formulation, secondary ingredients are included for transport stability and shelf-life.
Seamless alignment
The primary focus of formulation development at Intravacc is the seamless alignment with a vaccine’s intended administration route. Thus, our formulations are not only remarkably stable throughout storage life, but also perfectly suited for the method of delivery. Finally, the formulation is thoroughly tested in accelerated, real-time, and in-use stability studies. These studies are conducted with a careful selection of assays that characterize the performance and longevity of the formulation and, thus, warrant the vaccine’s potency and effectiveness from production to administration.
We offer high-throughput strategies combined with a design of experiments (DoE) approach for rapid identification of vaccine formulations. When optimizing liquid formulations and lyophilization processes, we provide insights into vaccine stability via accelerated and real-time programs. In addition, the (in-use) stability of your vaccine can be assessed for a wide range of delivery routes and dosage forms.
Alternative administration routes
The delivery route can be pivotal for the efficacy of a vaccine. Therefore, our experts are working on products and processes for alternative routes of administration beyond intramuscular injection, such as intranasal or mucosal sprays.
We are leaders in the development of intranasal vaccines
As a vaccination strategy, delivering antigen to the site of infection, in this case to the upper airways, has advantages:
- Mounting mucosal immunity in the respiratory tract
- Secretion of IgA in the mucosa of the upper airways enables effective neutralization of the virus or bacterium
- Lower shedding
- Activation of resident memory B and T cells in the respiratory mucosa elicits a quicker response
- The response can block subsequent infection
What can you expect from our formulation services?
- Short lead times
- All types of vaccine administration
- Accelerated and real-time stability plans supporting pre-clinical and clinical programs
Conjugation development
Conjugate vaccines merge non-immunogenic antigens, such as bacterial polysaccharides or short peptides, with protein-carrier antigens, thereby triggering, intensifying, and prolonging the immunological response. Conjugate vaccines offer speed, affordability, and high effectiveness, presenting an appealing alternative to traditional vaccines.
Proven expertise in conjugation
With two decades of experience, Intravacc excels in the design, development, and characterization of conjugate vaccines. Our Hib vaccine, for example, has been saving lives in India for years. More recently, Intravacc contributed to a project producing a semi-synthetic conjugate vaccine targeting Shigella flexneri 2a.
We have well-established and effective methods to purify polysaccharides and enable conjugation. Using advanced techniques, including high-performance liquid chromatography (HPLC), mass spectrometry, nuclear magnetic resonance (NMR), and colorimetric assays, we also rigorously assess every production step. Beyond classical conjugation, we are pioneering polysaccharide and peptide conjugation to outer membrane vesicles and synthetic polymers.
