Platforms
Technology
Proprietary platforms for vaccine development
Intravacc has developed several platforms to streamline the development of new vaccines. These platforms are the culmination of decades of successful vaccine innovation, and embody our knowledge and procedural blueprints for fast, efficient, and scalable vaccine design and development.
Outer Membrane Vesicle (OMV) platform
Intravacc is at the forefront of vaccine development for infectious diseases, antimicrobial resistance (AMR), and oncology leveraging our proprietary Outer Membrane Vesicle (OMV) platform.
Understanding Outer Membrane Vesicles (OMVs)
Outer Membrane Vesicles (OMVs) are naturally occurring, spherical particles, typically ranging in size from 20 to 200 nanometers. They are released by gram-negative bacteria and contain bacterial antigens.
OMV at a glance
- The OMV technology is based on the Outer Membrane Vesicles (OMVs) of gram-negative bacteria and is designed for the development of prophylactic vaccines.
- OMVs are spherical particles that are naturally released by gram-negative bacteria.
- Intravacc develops three types of OMV vaccines: homologous OMVs, heterologous OMVs, and click/mix OMVs.
- Corroborated by 84 scientific publications and covered by 8 patent families.
Application areas
- Bacterial, viral, and parasitic infection
- Antimicrobial resistance (AMR)
- Oncology
The advantages of OMVs:
- Non-replicating: OMVs do not replicate, making repeat vaccinations safer
- Proven safety: with LPS detoxification, OMVs have a solid safety profile
- Effective: intrinsically elicits a strong innate and adaptive immune response
- OMV Stability: maintain integrity, simplifying transport and long-term storage
- High yield: our platform has an optimized production efficiency
- High purity: our platform includes well-established and scalable purification steps
- Versatility: our platform allows combining OMVs with different antigen types
Intravacc's OMV Expertise
We back our OMV technology with expertise demonstrated through 84 scientific publications in peer-reviewed journals and 8 patent families since 2013. We’ve developed genetic tools to increase OMV yield, minimize toxicity, and tailor antigenic composition. Our scalable platform allows efficient antigen composition modifications through genetic manipulation or association with carrier OMVs. Several of our vaccines under development build on the OMV platform. Currently, we are actively working on the development of a vaccine for gonorrhea and COVID-19.
Using OMVs in Vaccines
Intravacc offers three types of OMV-based vaccines, each designed to target specific diseases and antigens:
Homologous OMVs:
These vaccines are derived directly from the target Gram-negative bacterium, offering broad protection by the natural incorporation of numerous antigens.
Heterologous OMVs:
For when direct production of OMVs is impractical, we have developed a technology to express antigens within our OMV platform that enables vaccine production.
Mix OMVs:
OMV Technology
A versatile new vaccine platform
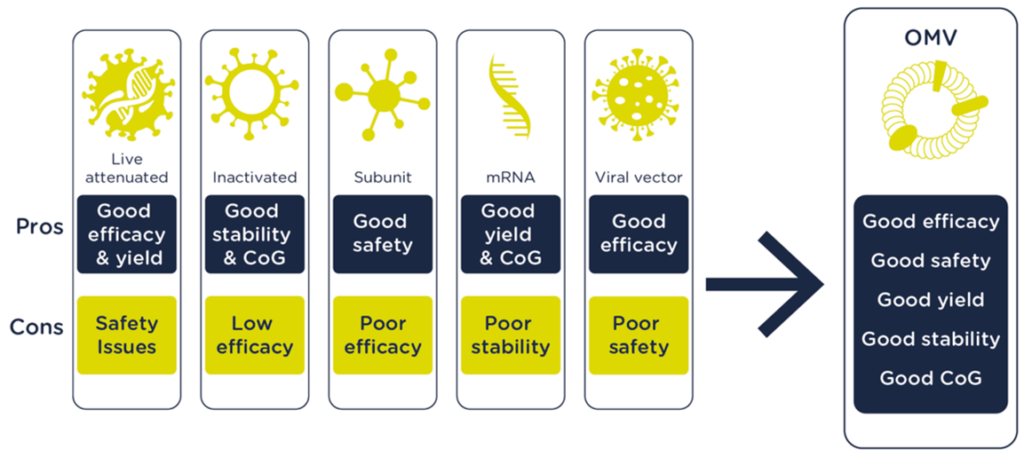
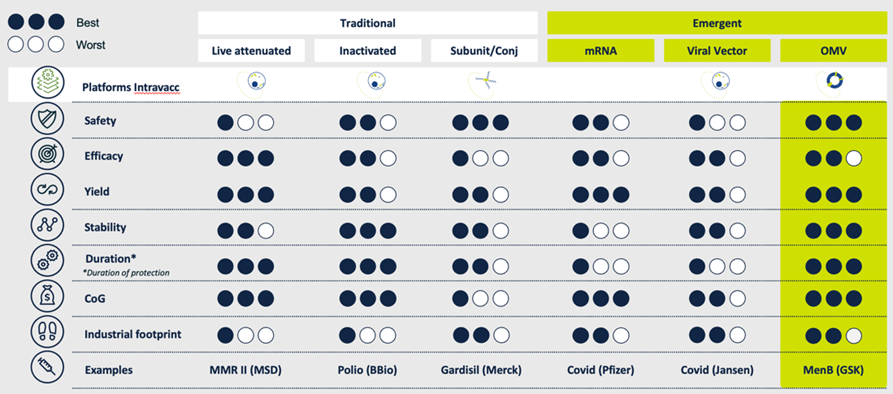
Truly exceptional among our vaccine platforms is our OMV technology. Outer Membrane Vesicles or OMVs have been explored for vaccines since the 1980s and our experience manipulating and optimizing these membrane particles to generate effective and safe vaccines extends back over a decade. We have a number of vaccine candidates that build on this technology, which offers numerous advantages over alternative platforms, both in terms of product performance and manufacturing.
OMV technology- and our CDMO services address vaccine development challenges
- Efficient use of materials and equipment: OMV-based vaccines reduce reagent and equipment use, like bacterial culture media and standard flow filters.
- Expert handling of process complexity: Our CDMO experts easily guide you through the creation of a new OMV, process optimization, stability, and standard monitoring assay development.
- Scalable manufacturing capacity: With over 10 years producing industrial OMV Clinical Trial Materials (CTM) in scales from 0.5 to 5 L, we offer the infrastructure and knowledge to achieve high yields in batch production for clinical studies.
What are OMVs exactly?
OMVs are 20-200 nm spherical particles that are naturally released by Gram-negative bacteria. They harbor many bacterial antigens and can be modified to display disease-specific antigens while increasing the safety of these vesicles by detoxification of LPS. These modified OMV have proven to be safe and intrinsically capable of stimulating both innate and adaptive immunity. Currently, vaccine candidates are developed in one of three different modes:
- Homologous OMVs, which are isolated directly from the disease pathogen.
- Heterologous OMVs that are designed to display disease-specific antigens.
- Mix OMVs, which contain externally added immunogenic peptides and/or proteins that can either by mixed with or linked to the OMV to drive an effective adaptive immunity.
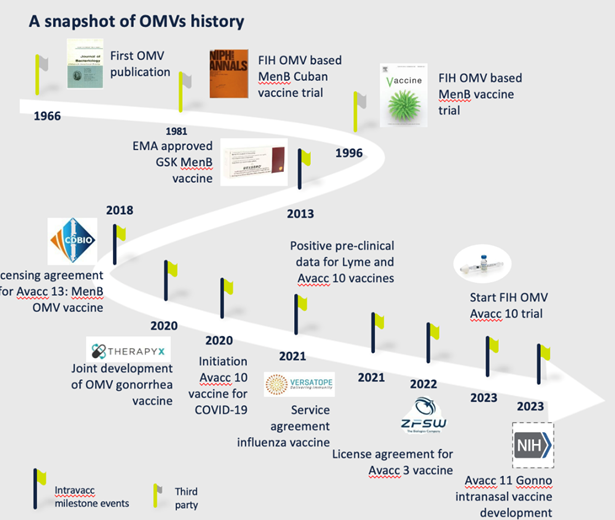
The history and evolution of OMV technology
The performance and safety of OMV technology as a platform for vaccines is well documented. Human studies conducted by others have shown that OMVs are safe for both intramuscular and intranasal immunization.
Research and development at Intravacc have led to over 80 scientific publications and nine patents.
Our own investigations using OMV technology span the completion of four pre-clinical candidates of which one has been tested in a phase I clinical study.
Intravacc is your trusted partner for OMV-based vaccine development
Our focus is on providing accurate, evidence-based solutions for scientists and researchers dedicated to addressing infectious diseases. Learn more about our OMV platform.
Matching CDMO services
From idea to GMP manufacturing, implement any of our production platforms with the support of our tailored CDMO services.
Our cell-based vaccine production platform
Our cell-based vaccine production platform offers a solid foundation for innovative vaccines for infectious diseases and oncolytic applications and is anchored by our Vero GMP cell line.
Cell-based at a glance
- The cell-based technology platform is used since 1987 by Intravacc (and its predecessors) to develop viral vaccines using the Vero cell line.
- Can be used for oncolytic, live-attenuated, and inactivated viral vaccines, as well as vector vaccines.
- Viral vaccine platforms are used globally for routine, small-, and large-scale manufacturing.
- Features patented technology.
Application areas
- Oncology
- Infectious diseases
Vero cells: a legacy of excellence
Vero cells, initially isolated and immortalized from the kidney of an African Green Monkey in the early 1960s, have evolved into the gold standard for viral vaccine production. They are renowned for their exceptional quality, yield, and safety.
An established and versatile cell-based platform
Intravacc has harnessed Vero cells for viral vaccine development since 1987.
Regulatory-approved Vero cell line
Our cGMP-grade, regulatory-approved Vero cell line forms the backbone of our viral vaccine production process. Clients worldwide rely on this platform for large-scale commercial vaccine manufacturing. Notably, we have successfully produced virus seed lots and clinical batches on Vero cells, including multiple generations of poliovirus, non-polio enteroviruses like EV71 and EV D68, and respiratory syncytial virus (RSV).
Vero cell platform
We’ve established the Vero cell platform for viral vaccine production processes at lab and pilot scales, facilitating rapid proof-of-concept development. Moreover, the platform is fully scalable, with Vero cells cultured on microcarriers in bioreactors, making them ideal for larger-scale production. We also employ downstream processes (DSP) to ensure vaccine purity through, for example, host cell protein removal. Finally, we provide ‘ready to use’ cGMP-grade cell banks and comprehensive technology transfer packages for immediate project initiation. Currently, most of our viral vaccine development projects are built on the Vero cell platform.
Learn more about our Vero cells
View which of vaccine candidates were build on Cell-based technology
Complementarity and expertise
With the Vero cell line at our disposal, Intravacc has the capability to propagate a wide range of viruses, whether from clinical isolates or through virus rescue from plasmid DNA.
Our commitment to scientific excellence and rigorous standards ensures that our cell-based vaccine production platform is a dependable resource for effective vaccine development.
Collaborate with us to advance your vaccine projects
Matching CDMO services
From idea to GMP manufacturing, implement any of our production platforms with the support of our tailored CDMO services.
Time-tested solutions
Intravacc’s 20-year legacy in the design, development, and characterization of conjugate vaccines speaks volumes about our commitment to combating diseases with time-tested solutions.
Conjugation at a glance
- Intravacc has decades of experience designing and developing conjugate vaccines.
- Conjugate vaccines are proven to be a fast, low-cost, and effective solution for bacterial infections.
- Features patented technology.
Application areas
- Infectious diseases
- Oncology (Therapeutic vaccines)
- CNS – Neuro-degeneration, for example for ALS (Therapeutic vaccines)
Conjugate vaccines: triggering immunity
Conjugate vaccines, hailed for their success in preventing bacterial infections, are engineered by covalently binding a bacterial polysaccharide or peptide—an agent that alone cannot induce immunological memory—to a protein carrier antigen. This fusion triggers a potent and sustained immunological response. We employ a range of carriers, including tetanus toxoid, diphtheria toxoid (CRM197 ), and Outer Membrane Vesicles (OMVs), to craft effective conjugate vaccines.
Intravacc expertise in production and more
Intravacc has honed the art of polysaccharide isolation and optimization, tailoring their length to maximize efficacy. Our arsenal includes versatile conjugation methods suitable for synthetic sugar antigens and peptides.
Services
Beyond production, we possess the proficiency to comprehensively characterize conjugate vaccines. We offer a suite of services to evaluate production processes and ensure vaccine quality. That suite includes fully defined vaccine batches that can meet the specific needs of both research and Good Manufacturing Practices (GMP) and advanced techniques for polysaccharide purification that ensure the purity of vaccine components. Our expertise extends to both upstream and downstream processing (USP and DSP). Coupled with analytical capabilities like HPLC (High-Performance Liquid Chromatography), mass spectrometry, NMR (Nuclear Magnetic Resonance), and colorimetric assays, we can meticulously evaluate and optimize every stage of the vaccine production process.
Learn more about our Conjugation platform.
A strong track record in conjugate vaccines
Our unwavering dedication to science and innovation empowers our partners in the creation of conjugate vaccine solutions that combat bacterial infections effectively and reliably. For example, Intravacc’s conjugate Hib vaccine has been saving lives in the Indian market for years. Our contributions to that development are substantiated by 6 published manuscripts and 1 patent family. More recently, we collaborated in a consortium to develop a semi-synthetic conjugate vaccine against Shigella flexnerii 2a. We led the process development and scale-up and subsequently produced the vaccine for phase I and II clinical studies. This vaccine is currently in phase II clinical trials.
See which of these vaccine candidates rely on Conjugation.
Partner with us to advance your vaccine projects
Matching CDMO services
From idea to GMP manufacturing, implement any of our production platforms with the support of our tailored CDMO services.
Escherichia coli expression system
The Escherichia coli expression system is routinely used in the production of proteins that require no eurkaryotic post-translational modifications. As an expression system, E. coli cultures are well-established, with time-tested growth and optimal performance in the production of antigens.
Our E.coli expression Technology is an essential tool in the production of recombinant proteins as vaccine components that can also be combined with the OMV or Conjugation platforms to develop novel and effective vaccine candidates.
E.coli expression at a glance
- E.coli expression is a well-established bacterial expression system for the production of targeted proteins that can be combined with OMVs or Conjugation.
- A fast, cost-effective, and robust platform for recombinant protein production that is used globally in vaccine manufacturing.
- Freedom to operate for certain applications.
Application areas
- Production of proteins of any kind
- Infectious diseases
The advantages of E.coli expression are clear:
- Speed: A fast, cost-effective, and versatile production platform.
- Reliability: Robust and high-productivity expression of recombinant proteins.
- Access: A universally used antigen production system.
- Relevant: Applied to protein expression for vaccines against cancer and infectious diseases.
Case study: an immunotherapy for solid tumors
We are working with CimCure to develop a cancer vaccine that targets blood vessels supplying solid cancers and thus prevents tumor growth.
Learn more about our work we are doing with E.coli expression
Matching CDMO services
From idea to GMP manufacturing, implement any of our production platforms with the support of our tailored CDMO services.
